Our Research
Recent Publications

Transient microglial absence assists postmigratory cortical neurons in proper differentiation.
Abstract
In the developing cortex, postmigratory neurons accumulate in the cortical plate (CP) to properly differentiate consolidating subtype identities. Microglia, despite their extensive surveying activity, temporarily disappear from the midembryonic CP. However, the mechanism and significance of this absence are unknown. Here, we show that microglia bidirectionally migrate via attraction by CXCL12 released from the meninges and subventricular zone and thereby exit the midembryonic CP. Upon nonphysiological excessive exposure to microglia in vivo or in vitro, young postmigratory and in vitro-grown CP neurons showed abnormal differentiation with disturbed expression of the subtype-associated transcription factors and genes implicated in functional neuronal maturation. Notably, this effect is primarily attributed to interleukin 6 and type I interferon secreted by microglia. These results suggest that "sanctuarization" from microglia in the midembryonic CP is required for neurons to appropriately fine-tune the expression of molecules needed for proper differentiation, thus securing the establishment of functional cortical circuit.

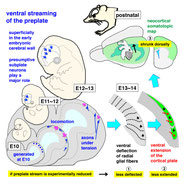
Dorsal-to-Ventral Cortical Expansion Is Physically Primed by Ventral Streaming of Early Embryonic Preplate Neurons.
Abstract
Despite recent studies elucidating the molecular mechanisms underlying cortical patterning and map formation, very little is known about how the embryonic pallium expands ventrally to form the future cortex and the nature of the underlying force-generating events. We find that neurons born at embryonic day 10 (E10) in the mouse dorsal pallium ventrally stream until E13, thereby superficially spreading the preplate, and then constitute the subplate from E14. From E11 to E12, the preplate neurons migrate, exerting pulling and pushing forces at the process and the soma, respectively. At E13, they are morphologically heterogeneous, with ∼40% possessing corticofugal axons, which are found to be in tension. Ablation of these E10-born neurons attenuates both deflection of radial glial fibers (by E13) and extension of the cortical plate (by E14), which should occur ventrally, and subsequently shrinks the postnatal neocortical map dorsally. Thus, the preplate stream physically primes neocortical expansion and arealization.
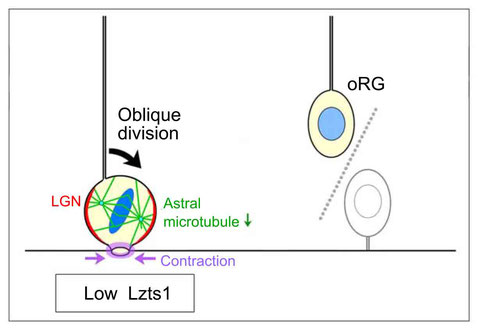
Lzts1 controls both neuronal delamination and outer radial glial-like cell generation during mammalian cerebral development.
Abstract
In the developing central nervous system, cell departure from the apical surface is the initial and fundamental step to form the 3D, organized architecture. Both delamination of differentiating cells and repositioning of progenitors to generate outer radial glial cells (oRGs) contribute to mammalian neocortical expansion; however, a comprehensive understanding of their mechanisms is lacking. Here, we demonstrate that Lzts1, a molecule associated with microtubule components, promotes both cell departure events. In neuronally committed cells, Lzts1 functions in apical delamination by altering apical junctional organization. In apical RGs (aRGs), Lzts1 expression is variable, depending on Hes1 expression levels. According to its differential levels, Lzts1 induces diverse RG behaviors: planar division, oblique divisions of aRGs that generate oRGs, and their mitotic somal translocation. Loss-of-function of lzts1 impairs all these cell departure processes. Thus, Lzts1 functions as a master modulator of cellular dynamics, contributing to increasing complexity of the cerebral architecture during evolution.

Embryonic neocortical microglia express Toll-like receptor 9 and respond to plasmid DNA injected into the ventricle: technical considerations regarding microglial distribution in electroporated brain walls.
- 1
- Department of Anatomy and Cell Biology, Nagoya University Graduate School of Medicine, Nagoya 466-8550, Japan.
Abstract
Microglia, the resident immune cells in the CNS, play multiple roles during development. In the embryonic cerebral wall, microgliamodulate the functions of neural stem/progenitor cells through their distribution in regions undergoing cell proliferation and/or differentiation. Previous studies using CX3CR1-GFP transgenic mice demonstrated that microglia extensively survey these regions. To simultaneously visualize microglia and neural-lineage cells that interact with each other, we applied the in utero electroporation (IUE) technique, which has been widely used for gene-transfer in neurodevelopmental studies, to CX3CR1-GFP mice (males and females). However, we unexpectedly faced a technical problem: although microglia are normally distributed homogeneously throughout the mid-embryonic cortical wall with only limited luminal entry, the intraventricular presence of exogenously derived plasmid DNAs induced microglia to accumulate along the apical surface of the cortex and aggregate in the choroid plexus. This effect was independent of capillary needle puncture of the brain wall or application of electrical pulses. The microglial response occurred at plasmid DNAconcentrations lower than those routinely used for IUE, and was mediated by activation of Toll-like receptor 9 (TLR9), an innate immune sensor that recognizes unmethylated cytosine-phosphate guanosine motifs abundant in microbial DNA. Administration of plasmid DNAtogether with oligonucleotide 2088, the antagonist of TLR9, partially restored the dispersed intramural localization of microglia and significantly decreased luminal accumulation of these cells. Thus, via TLR9, intraventricular plasmid DNA administration causes aberrant distribution of embryonic microglia, suggesting that the behavior of microglia in brain primordia subjected to IUE should be carefully interpreted.
Microglia extensively survey the developing cortex via the CXCL12/CXCR4 system to help neural progenitors to acquire differentiated properties.
Abstract
Neocortical development proceeds through the formation of new zones in which neural-lineage cells are organized based on their differentiation status. Although microglia initially distribute homogeneously throughout the growing cerebral wall, they accumulate in the inner cytogenic zone, the ventricular zone (VZ) and the subventricular zone (SVZ) in the mid-embryonic stage. However, the roles of these cells remain to be elucidated. In this study, we found that microglia, despite being only a minor population of the cells that constitute the cerebral wall, promote the differentiation of neural progenitor cells by frequently moving throughout the cortex; their migration is mediated by the CXCL12/CXCR4 system. Pulse-chase experiments confirmed that microglia help Pax6+ stem-like cells to differentiate into Tbr2+ intermediate progenitors. Further, monitoring of microglia by live imaging showed that administration of AMD3100, an antagonist of CXCR4, dampened microglial movement and decreased microglial surveillance throughout the cortex. In particular, arrest of microglial motion led to a prominent decrease in the abundance of Tbr2+ cells in the SVZ. Based on our findings, we propose that extensive surveillance by microglia contributes to the efficient functioning of these cells, thereby regulating the differentiation of neural stem-like cells.

Development. June 26 2018, dev.162883.
DOI: 10.1242/dev.162883
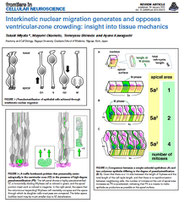
Differentiating cells mechanically limit progenitor cells’ interkinetic nuclear migration to secure apical cytogenesis
Abstract
Many proliferative epithelia are pseudostratified due to cell cycle-dependent interkinetic nuclear migration (IKNM, basal during G1 and apicalduring G2). Although most epithelia, including early embryonic neuroepithelia (≤100 µm thick), undergo IKNM over the entire apicobasal extent, more apicobasally elongated (300 µm) neural progenitor cells (also called "radial glia") in the mid-embryonic mouse cerebral wall move their nuclei only within its apical (100 µm) compartment, leaving the remaining basal part nucleus-free (fiber-like). How this IKNM range (i.e., the thickness of a pseudostratified "ventricular zone" [VZ]) is determined remains unknown. Here, we report external fencing of IKNM and VZ by differentiating cells. When a tight stack of multipolar cells just basal to VZ was "drilled" via acute neuron-directed expression of diphtheria toxin, IKNM of apicobasally connected progenitor cells continued far basally (200 µm). The unfencing-induced, basally overshot nuclei stay in S phase too long and do not move apically, suggesting that external limitation of IKNM is necessary for progenitors to undergo normal cytogenetic behaviors. Thus, physical collaboration between progenitors and differentiating cells including neurons underlies brain development.
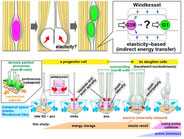
Elasticity-based boosting of neuroepithelial nucleokinesis via indirect energy transfer from mother to daughter.
Abstract
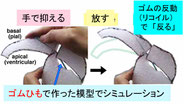
Neural progenitor cells (NPCs), which are apicobasally elongated and densely packed in the developing brain, systematically move their nuclei/somata in a cell cycle-dependent manner, called interkinetic nuclear migration (IKNM): apical during G2 and basal during G1. Although intracellular molecular mechanisms of individual IKNM have been explored, how heterogeneous IKNMs are collectively coordinated is unknown. Our quantitative cell-biological and in silico analyses revealed that tissue elasticity mechanically assists an initial step of basalward IKNM. When the soma of an M-phase progenitor cell rounds up using actomyosin within the subapical space, a microzone within 10 μm from the surface, which is compressed and elastic because of the apical surface's contractility, laterally pushes the densely neighboring processes of non-M-phase cells. The pressed processes then recoil centripetally and basally to propel the nuclei/somata of the progenitor's daughter cells. Thus, indirect neighbor-assisted transfer of mechanical energy from mother to daughter helps efficient brain development.
Neural Progenitor Cells Undergoing Yap/Tead-Mediated Enhanced Self-Renewal Form Heterotopias More Easily in the Diencephalon than in the Telencephalon.
Abstract
Spatiotemporally ordered production of cells is essential for brain development. Normally, most undifferentiated neural progenitor cells (NPCs) face the apical (ventricular) surface of embryonic brain walls. Pathological detachment of NPCs from the apical surface and their invasion of outer neuronal territories, i.e., formation of NPC heterotopias, can disrupt the overall structure of the brain. Although NPC heterotopias have previously been observed in a variety of experimental contexts, the underlying mechanisms remain largely unknown. Yes-associated protein 1 (Yap1) and the TEA domain (Tead) proteins, which act downstream of Hippo signaling, enhance the stem-like characteristics of NPCs. Elevated expression of Yap1 or Tead in the neural tube (future spinal cord) induces massive NPC heterotopias, but Yap/Tead-induced expansion of NPCs in the developing brain has not been previously reported to produce NPC heterotopias. To determine whether NPC heterotopias occur in a regionally characteristic manner, we introduced the Yap1-S112A or Tead-VP16 into NPCs of the telencephalon and diencephalon, two neighboring but distinct forebrain regions, of embryonic day 10 mice by in utero electroporation, and compared NPC heterotopia formation. Although NPCs in both regions exhibited enhanced stem-like behaviors, heterotopias were larger and more frequent in the diencephalon than in the telencephalon. This result, the first example of Yap/Tead-induced NPC heterotopia in the forebrain, reveals that Yap/Tead-induced NPC heterotopia is not specific to the neural tube, and also suggests that this phenomenon depends on regional factors such as the three-dimensional geometry and assembly of these cells.

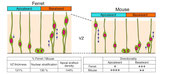
Differences in the Mechanical Properties of the Developing Cerebral Cortical Proliferative Zone between Mice and Ferrets at both the Tissue and Single-Cell Levels.
Abstract
Cell-producing events in developing tissues are mechanically dynamic throughout the cell cycle. In many epithelial systems, cells are apicobasally tall, with nuclei and somata that adopt different apicobasal positions because nuclei and somata move in a cell cycle-dependent manner. This movement is apical during G2 phase and basal during G1 phase, whereas mitosis occurs at the apical surface. These movements are collectively referred to as interkinetic nuclear migration, and such epithelia are called "pseudostratified." The embryonic mammalian cerebral cortical neuroepithelium is a good model for highly pseudostratified epithelia, and we previously found differences between mice and ferrets in both horizontal cellular density (greater in ferrets) and nuclear/somal movements (slower during G2 and faster during G1 in ferrets). These differences suggest that neuroepithelial cells alter their nucleokinetic behavior in response to physical factors that they encounter, which may form the basis for evolutionary transitions toward more abundant brain-cell production from mice to ferrets and primates. To address how mouse and ferret neuroepithelia may differ physically in a quantitative manner, we used atomic force microscopy to determine that the vertical stiffness of their apical surface is greater in ferrets (Young's modulus = 1700 Pa) than in mice (1400 Pa). We systematically analyzed factors underlying the apical-surface stiffness through experiments to pharmacologically inhibit actomyosin or microtubules and to examine recoiling behaviors of the apical surface upon laser ablation and also through electron microscopy to observe adherens junction. We found that although both actomyosin and microtubules are partly responsible for the apical-surface stiffness, the mouse<ferret relationship in the apical-surface stiffness was maintained even in the presence of inhibitors. We also found that the stiffness of single, dissociated neuroepithelial cells is actually greater in mice (720 Pa) than in ferrets (450 Pa). Adherens junction was ultrastructurally comparable between mice and ferrets. These results show that the horizontally denser packing of neuroepithelial cell processes is a major contributor to the increased tissue-level apical stiffness in ferrets, and suggest that tissue-level mechanical properties may be achieved by balancing cellular densification and the physical properties of single cells.
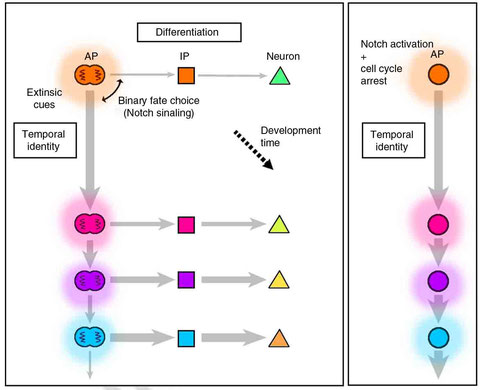
Cell-cycle-independent transitions in temporal identity of mammalian neural progenitor cells.
Abstract
During cerebral development, many types of neurons are sequentially generated by self-renewing progenitor cells called apical progenitors (APs). Temporal changes in AP identity are thought to be responsible for neuronal diversity; however, the mechanisms underlying such changes remain largely unknown. Here we perform single-cell transcriptome analysis of individual progenitors at different developmental stages, and identify a subset of genes whose expression changes over time but is independent of differentiation status. Surprisingly, the pattern of changes in the expression of such temporal-axis genes in APs is unaffected by cell-cycle arrest. Consistent with this, transient cell-cycle arrest of APs in vivo does not prevent descendant neurons from acquiring their correct laminar fates. Analysis of cultured APs reveals that transitions in AP gene expression are driven by both cell-intrinsic and -extrinsic mechanisms. These results suggest that the timing mechanisms controlling AP temporal identity function independently of cell-cycle progression and Notch activation mode.

The neuroepithelium (NE) or ventricular zone (VZ), from which multiple types of brain cells arise, is pseudostratified. In the NE/VZ, neural progenitor cells are elongated along the apicobasal axis, and their nuclei assume different apicobasal positions. These nuclei move in a cell cycle–dependent manner, i.e., apicalward during G2 phase and basalward during G1 phase, a process called interkinetic nuclear migration (INM). This review will summarize and discuss several topics: the nature of the INM exhibited by neural progenitor cells, the mechanical difficulties associated with INM in the developing cerebral cortex, the community-level mechanisms underlying collective and efficient INM, the impact on overall brain formation when NE/VZ is overcrowded due to loss of INM, and whether and how neural progenitor INM varies among mammalian species. These discussions will be based on recent findings obtained in live, three-dimensional specimens using quantitative and mechanical approaches. Experiments in which overcrowding was induced in mouse neocortical NE/VZ, as well as comparisons of neocortical INM between mice and ferrets, have revealed that the behavior of NE/VZ cells can be affected by cellular densification. A consideration of the physical aspects in the NE/VZ and the mechanical difficulties associated with high-degree pseudostratification is important for achieving a better understanding of neocortical development and evolution.
Neurogenin2-d4Venus and Gadd45g-d4Venus transgenic mice: Visualizing mitotic and migratory behaviors of cells committed to the neuronal lineage in the developing mammalian brain
Development, Growth & Differentiation 56, 293-304, 2014
To achieve highly sensitive and comprehensive assessment of the morphology and dynamics of cells committed to the neuronal lineage in mammalian brain primordia, we generated two transgenic mouse lines expressing a destabilized (d4) Venus controlled by regulatory elements of the Neurogenin2 (Neurog2) or Gadd45g gene. In mid-embryonic neocortical walls, expression of Neurog2-d4Venus mostly overlapped with that of Neurog2 protein, with a slightly (1 h) delayed onset. Although Neurog2-d4Venus and Gadd45g-d4Venus mice exhibited very similar labeling patterns in the ventricular zone (VZ), in Gadd45g-d4Venus mice cells could be visualized in more basal areas containing fully differentiated neurons, where Neurog2-d4Venus fluorescence was absent. Time-lapse monitoring revealed that most d4Venus+ cells in the VZ had processes extending to the apical surface; many of these cells eventually retracted their apical process and migrated basally to the subventricular zone, where neurons, as well as the intermediate neurogenic progenitors that undergo terminal neuron-producing division, could be live-monitored by d4Venus fluorescence. Some d4Venus+ VZ cells instead underwent nuclear migration to the apical surface, where they divided to generate two d4Venus+ daughter cells, suggesting that the symmetric terminal division that gives rise to neuron pairs at the apical surface can be reliably live-monitored. Similar lineage-committed cells were observed in other developing neural regions including retina, spinal cord, and cerebellum, as well as in regions of the peripheral nervous system such as dorsal root ganglia. These mouse lines will be useful for elucidating the cellular and molecular mechanisms underlying development of the mammalian nervous system.

Ferret-mouse differences in interkinetic nuclear migration and cellular densification in the neocortical ventricular zone.
Neuroscience Research 83, 25-32, 2014
The thick outer subventricular zone (OSVZ) is characteristic of the development of human neocortex. How this region originates from the ventricular zone (VZ) is largely unknown. Recently, we showed that over-proliferation–induced acute nuclear densification and thickening of the VZ in neocortical walls of mice, which lack an OSVZ, causes reactive delamination of undifferentiated progenitors and invasion by these cells of basal areas outside the VZ. In this study, we sought to determine how VZ cells behave in non-rodent animals that have an OSVZ. A comparison of mid-embryonic mice and ferrets revealed: (1) the VZ is thicker and more pseudostratified in ferrets. (2) The soma and nuclei of VZ cells were horizontally and apicobasally denser in ferrets. (3) Individual endfeet were also denser on the apical (ventricular) surface in ferrets. (4) In ferrets, apicalward nucleokinesis was less directional, whereas basalward nucleokinesis was more directional; consequently, the nuclear density in the periventricular space (within 16 μm of the apical surface) was smaller in ferrets than in mice, despite the nuclear densification seen basally in ferrets. These results suggest that species-specific differences in nucleokinesis strategies may have evolved in close association with the magnitudes and patterns of nuclear stratification in the VZ.

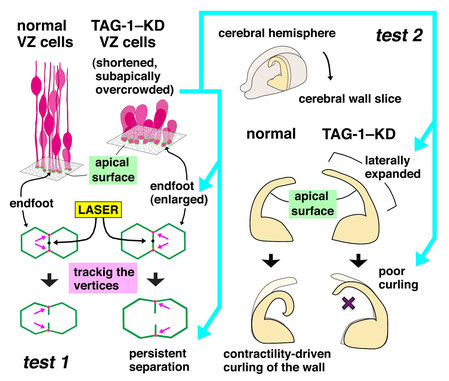
TAG-1–assisted progenitor elongation streamlines nuclear migration to optimize subapical crowding.
Nat. Neurosci. 16, 1556-1566, 2013, DOI: 10.1038/nn.3525 PubMed
Neural progenitors exhibit cell cycle–dependent interkinetic nuclear migration (INM) along the apicobasal axis. Despite recent advances in understanding its
underlying molecular mechanisms, the processes to which INM contributes mechanically and the regulation of INM by the apicobasally elongated morphology of progenitors remain unclear. We found
that knockdown of the cell-surface molecule TAG-1 resulted in retraction of neocortical progenitors' basal processes. Highly shortened stem-like progenitors failed to undergo basalward INM and
became overcrowded in the periventricular (subapical) space. Surprisingly, the overcrowded progenitors left the apical surface and migrated into basal neuronal territories. These observations,
together with the results of in toto imaging and physical tests, suggest that progenitors may sense and respond to excessive mechanical stress. Although, unexpectedly, the
heterotopic progenitors remained stem-like and continued to sequentially produce neurons until the late embryonic period, histogenesis was severely disrupted. Thus, INM is essential for
preventing overcrowding of nuclei and their somata, thereby ensuring normal brain histogenesis.
Results
・Heterogeneous apicobasal nuclear movements in the VZ
・TAG-1 knockdown induces progenitor shortening
・Shortened progenitors are periventricularly overcrowded
・Overcrowded cells are under excessive mechanical stress
・Overcrowded progenitors delaminate and form heterotopia
・Heterotopia maintains cytogenesis but disrupt histogenesis
Discussion
・TAG-1 and progenitors' histogenetic behaviors
・Morphology-based and synecological understanding of INM
・Mechanics underlying progenitors' behaviors
・Robust cytogenesis by shortened and heterotopic progenitors

Ongoing Research Project:
Neurogenesis regulated through three-dimensional cellular movement and cell-cell interactions within the neuroepithelium
Supported by Grant-in-Aid for Scientific Research on Innovative Areas (Cross-talk between moving cells and microenvironment as a basis of emerging order in multicellular system, FY2010-2014)
The neural tube and the walls of the early embryonic brain vesicles are composed entirely of undifferentiated progenitor cells and are referred to collectively as the neuroepithelium (NE). Structurally, the NE is pseudostratified; that is, although there may be up to ten layers of nuclei, the cytoplasm of each cell extends to contact both the apical and basal surfaces of the wall, resulting in a bipolar cellular morphology up to 100 μm in length. Progenitor cells are born at the apical surface of the NE, and their nuclei move toward the basal side of the NE during G1 of the cell cycle. After completing S-phase in the basal portion of the NE, the nuclei return to the apical surface, where they undergo division as their parent cells did. Thus, the location of any given progenitor cell during this interkinetic nuclear migration (INM) reflects the age of the cell or its degree of progression through the cell cycle. In this project, we will carefully observe cells within the NE, focusing on the relationship between cells differing in age, cell cycle status, and migration direction, and will perform functional experiments to manipulate cell-cell interactions. The goal of this project is to understand the significance of INM and pseudostratification in ordered neurogenesis.
Previous Researches
Migration, early axonogenesis, and Reelin-dependent layer-forming behavior of early/posterior-born Purkinje cells in the developing mouse lateral cerebellum. Neural Dev. 5, 23 (2010)
Purpose
How the young Purkinje cells migrate and initiate the layer formation in response to Reelin in the developing cerebellum? Although the dependence of Purkinje cells’ layer formation on the secreted protein Reelin is well known and a prevailing model suggests that Purkinje cells migrate along the “radial glial” fibers connecting the ventricular and pial surfaces, it is not clear how Purkinje cells behave in response to Reelin to initiate their layer. Furthermore, it is not known what nascent Purkinje cells look like in vivo. When and how Purkinje cells start axonogenesis must also be elucidated.
Results
We found that Purkinje cells generated
on embryonic day (E) 10 in the developing mouse cerebellum migrate tangentially towards the anterior, exhibiting an elongated morphology consistent with axonogenesis at E12. After their somata
reach the outer/dorsal region by E13, they change “posture” by E14 through remodeling of non-axon (dendrite-like) processes and a switchback-like mode of somal movement towards a superficial
Reelin-rich zone, while their axon-like fibers remain relatively deep, which demarcates the somata-packed portion as a plate (called the Purkinje plate). In the cerebellum
of reelermice, which suffer from ataxic gait due to abnormal cerebellar cortical histogenesis, the early born posterior lateral Purkinje cells are initially normal during migration
with anteriorly-extended axon-like fibers until E13, but then fail to form the plate due to the lack of the posture-change step. This is the first
demonstration of the beginning of layer formation by Purkinje cells (summarized in the figure, published in Neural Development [2010] and selected as a “highly accessed
paper”). This study provides a solid basis for further elucidation of Reelin’s function and the mechanisms underlying the cerebellar
corticogenesis, and will contribute to the understanding of how polarization of individual cells drives the overall brain morphogenesis. Our finding is relevant to future efforts to reconstruct
the Purkinje cells layer in the cerebellum affected by degenerative diseases.
Rac is involved in the interkinetic nuclear migration of cortical progenitor cells. Neurosci. Res.63, 294-301 (2009)
Purpose
In the developing brain wall, neural progenitor cells exhibit nuclear migration during their cell cycle progression. After completing S phase in the basal (pial) side of the neuroepithelium or ventricular zone (VZ), their nuclei go to the apical (ventricular) surface of the VZ, where they undergo division. The nuclei of cells newly born at the apical surface of the VZ move toward the basal side during G1 phase of the cell cycle. This to-and-fro nuclear/somal movement or interkinetic nuclear migration (INM) was first suggested by Sauer in 1935 and was experimentally proven by pulse-and-chase experiments based on 3H-thymidine labeling. Our group previously established a time-lapse monitoring system of INM (Neuron, 2001). Although INM seems to be important for proper cytogenesis, the molecular mechanisms that underlie INM are not well understood. The small GTPase Rac functions in a number of cellular processes, including cytoskeletal regulation, cell-cell adhesion, and migration. Recent three-dimensional functional studies in the developing brain have shown that Rac regulates migration of neurons and extension of neuronal processes. Based on the function of Rac in neurons that exhibit dynamic morphological changes, we reasoned that Rac might also work in progenitor cells, which show highly dynamic behavior, such as INM and cell division. Involvement of Rac in INM has not yet been directly assessed at the single-cell level.
Results
By cross-sectional and orthogonal immunofluorescence examination, we found that Rac1 is expressed in mid-embryonic mouse telencephalic progenitor cells. Localization is marked at the apical endfoot. Pharmacological inhibition of Rac in slice cultures during the adventricular phase of INM retards nucleokinesis and results in unsuccessful cytokinesis at the apical surface. Similar results were obtained by introducing a dominant-negative form of Rac1. These results suggest that Rac may play a role in INM in the developing mouse brain (Neuroscience Research, 2009).

Periventricular Notch activation and asymmetric Ngn2 and Tbr2 expression in pair-generated neocortical daughter cells. Mol. Cell. Neurosci. 40, 225-233 (2009)
Purpose
Formation of the neocortex relies on the precise balance between neurogenesis and maintenance of a neural progenitor pool in the pallial primordium during embryonic development. The mechanism of this asymmetric daughter-cell production by the progenitor population has been extensively studied, but is still not fully understood In order to elucidate the intrinsic and extrinsic mechanisms regulating the asymmetric cell output of progenitor cells, a careful analysis of the spatiotemporal expression patterns of factors controlling cell fate is needed. Especially, how Notch is activated in neural progenitor cells and their daughter cells and how Notch ligand such as Delta-like 1 (Dll1) is expressed need to be elucidated. We previously found that differentiation of progenitor cells towards the neuronal lineage is regulated by Neurogenin2 (Ngn2) (Development, 2004). Then, Tbr2 was reported to be also important for the commitment to the neuronal lineage. Therefore, we extended our analysis to ask how nascent daughter cells start expressing Ngn2, and to determine the relationship between Ngn2 and Tbr2 during the course of lineage commitment of neural progenitor cells.
Results
Through collaboration with Dr. Yoon Kong (Pohang University of Science and Technology), we found that neural progenitor cells committed to the neuronal lineage expresse the Notch-ligand Delta-like 1 (Dll1). Further, our time-lapse observation directly showed that progenitor cells whose Notch activation is reduced by conditional knock-out of Mind bomb-1 (Mib1), an essential component of Notch ligand endocytosis, failed to maintain undifferentiated progenitor pool, leading to premature neuronal differentiation (Neuron, 2008). To track the developmental time course of Ngn2 and Tbr2 expression in VZ cells, time-lapse observation was performed on daughter cells of individually DiI-labeled progenitor cells in cultured neocortical slices, followed by immunostaining for Ngn2 or Tbr2. We found that Ngn2 protein expression was initiated asymmetrically in the surface-generated daughter cells as early as 2 h after birth, about 2 h earlier than the onset of Tbr2 expression. Luciferase and ChIP assays further revealed that Tbr2 is directly downstream of Ngn2. Daughter cells expressing Ngn2 or Tbr2 were connected to the ventricular surface and maintained expression of the two transcription factors after detaching from the ventricular surface. Inhibition of Notch signaling in nascent surface-born daughter cells by treatment with a γ-seceretase inhibitor strikingly increased the frequency of Ngn2 expression in daughter cells 2 h after birth. Activation of Notch was observed not only in the basal VZ, but also in the periventricular VZ containing nascent daughter cells. These results suggest that the periventricular area and the initial morphology of surface-born daughter cells may be important for the regulation of cell fate choice. (Molecular Cellular Neuroscience, 2009).


Publications (2004~, Miyata lab @ Nagoya)
Okamoto, M., Shinoda, T., Kawaue, T., Nagasaka, A., Miyata, T. Ferret-mouse differences in interkinetic nuclear migration and cellular densification in the neocortical ventricular zone. Neurosci. Res. 83, 25-32, 2014
Kawaue T, Sagou K, Kiyonari H, Ota K, Okamoto M, Shinoda T, Kawaguchi A, Miyata T. Neurogenin2-d4Venus and Gadd45g-d4Venus transgenic mice: Visualizing mitotic and migratory behaviors of cells committed to the neuronal lineage in the developing mammalian brain. Dev Growth Differ. 56, 293-304, 2014
Namba, T., Kibe, Y., Funahashi, Y., Nakamuta, S., Takano, T., Ueno, T., Shimada, A., Kozawa, S., Okamoto, M., Shimoda, Y., Oda, K., Wada, Y., Masuda, T., Sakakibara, A., Igarashi, M., Miyata, T., Faivre-Sarrailh, C., Takeuchi, K., Kaibuchi, K. Pioneering axons regulate neuronal polarization in the devveloping cerebral cortex. Neuron 81, 814-829, 2014
Ageta-Ishihara, N., Miyata, T., Ohshima, C., Watanabe, M., Sato, Y., Hamamura, Y., Higashijima, T., Mazitschek, R., Bito, H., Kinoshita, M. Septins promote dendrite and axon development by negatively regulating microtubule stability via HDAC6-mediated deacetylation. Nat. Commun. 4: 2532, DOI: 10.1038/ncomms3532
Okamoto, M., Namba, T., Shinoda, T., Kondo, T., Watanabe, T., Inoue, Y., Takeuchi, K., Enomoto, Y., Ota, K., Oda, K., Wada, Y., Sagou, K., Saito, K., Sakakibara, A., Kawaguchi, A., Nakajima, K., Adachi, T., Fujimori, T., Ueda, M. Hayashi, S., Kaibuchi, K., Miyata, T. TAG-1–assisted progenitor elongation streamlines nuclear migration to optimize subapical crowding. Nat. Neurosci.,16: 1556-1566 (2013) DOI: 10.1038/nn.3525
Sakakibara, A., Ando, R., Sapir, T., Tanaka, T. Microtubule dynamics in neuronal morphogenesis. (2013) Open Biol. 3:130061 DOI: 10.1098/rsob.130061
Sapir, T., Levy, T., Sakakibara, A., Rabinkov, A., Miyata, T., Reiner, O. Shootin1 acts in concert with KIF20B to promote polarization of migrating neurons. (2013) J. Neurosci. 33:11932-11948 DOI: 10.1523/JNEUROSCI.5425-12.2013
Wu, J , Liu, L , Matsuda, T , Zao, Y, Rebane, A , Drobizhev, M , Chang, Y-F , Araki, S , Arai, Y , March, K , Thomas, HE, Sagou, K , Miyata, T, Nagai, T , Li, W-H , and Campbell, RE Improved orange and red Ca2+ indicators and photophysical considerations for optogenetic applications. ACS Chem. Neurosci. (2013.3.1 on line)DOI: 10.1021/cn400012b
Sakakibara, A.(corresponding author), Sato, T., Ando, R., Noguchi, N., Masaoka, M., Miyata, T. Dynamics of centrosome translocation and microtubule organization in neocortical neurons during distinct modes of polarization.Cereb. Cortex 2013 Jan 10. (doi:10.1093/cercor/bhs411)
Xie, M.-J., Yagi, H., Kuroda, K., Wang, C.-C., Komada, M., Zhao, H., Sakakibara, A., Miyata, T. Nagata, K., Iguchi, T., Sato, M. WAVE2-Abi2 complex controls growth cone activity and regulates the multipolar-bipolar transition as well as the initiation of glia-guided migration. Cereb. Cortex 23:1410-1423 (2013) (doi: 10.1093/cercor/bhs123)
Pérez-Martínez, F.J., Luque-Río, A., Sakakibara, A., Hattori, M., Miyata,
T., Luque, J. M. Reelin-dependent ApoER2 downregulation uncouples newborn neurons from progenitor cells. Biol. Open 1:1258-1263 (2012)
Nakamuta, S., Funahashi, Y., Namba, T., Arimura, N., Picciotto, M.R., Tokumitsu, H., Soderling, T.R., Sakakibara, A., Miyata, T., Kamiguchi, H., Kaibuchi, K. Local application of neurotrophins specifies axons through inositol 1,4,5-trisphosphate, calcium, and ca2+/calmodulin-dependent protein kinases. Sci. Signal. 4(199):ra76 (2011)
Natsume, S., Kato, T., Kinjo, S., Enomoto, A., Toda, H., Shimato, S., Ohka, F., Motomura, K., Kondo, Y., Miyata, T., Takahashi, M., Wakabayashi, T. Girdin maintains the stemness of glioblastoma stem cells. Oncogene 31, 2715-2724 (2011)
Miyata, T., Ono Y, Okamoto M, Masaoka M, Sakakibara A, Kawaguchi A, Hashimoto M, Ogawa M. Migration, early axonogenesis, and Reelin-dependent layer-forming behavior of early/posterior-born Purkinje cells in the developing mouse lateral cerebellum. Neural Dev. 5, 23 (2010)
![]()
Miyata, T., Kawaguchi, D., Kawaguchi, A., Gotoh, Y. Mechanisms that regulate the number of neurons during mouse neocortical development. Curr. Opin. Neurobiol. 20, 22-28 (2010)
Kato TM, Kawaguchi A, Kosodo Y, Niwa H, *Matsuzaki F.
Lunatic fringe potentiates Notch signaling in the developing brain. Mol Cell Neurosci. 45, 12-25, 2010
Uchida T, Baba A, Perez-Martinez FJ, Hibi T, Miyata T, Luque JM, Nakajima K, Hattori M. Downregulation of functional Reelin receptors in projection neurons implies that primary Reelin action occurs at early/premigratory stages. J Neurosci. 29:10653-62 (2009)
Saito K, Dubreuil V, Arai Y, Wilsch-Brauninger M, Schwudke D, Saher G, Miyata T, Breier G, Thiele C, Shevchenko A, Nave KA, Huttner WB. Ablation of cholesterol biosynthesis in neural stem cells increases their VEGF expression and angiogenesis but causes neuron apoptosis. Proc Natl Acad Sci U S A 106(20):8350-5 (2009)
Minobe, S., Sakakibara, A., Ohdachi, T., Kanda, R., Kimura, M., Nakatani, S., Tadokoro, R., Ochiai, W., Nishizawa, Y., Mizoguchi, A., Kawauchi, T., Miyata, T.: Rac is involved in the interkinetic nuclear migration of cortical progenitor cells. Neurosci. Res. 63, 294-301 (2009)
Ochiai, W.,* Nakatani, S.,* Takahara, T., Kainuma, M., Masaoka, M., Minobe, S., Namihira, M., Nakashima, K., Sakakibara, A., Ogawa, M., Miyata, T.: Periventricular Notch activation and asymmetric Ngn2 and Tbr2 expression in pair-generated neocortical daughter cells. Mol. Cell. Neurosci. 40, 225-233 (2009) (*Equal contribution)
Yoon, K.-J., Koo, B.-K., Jeong, H.-W., Ghim, J., Kwon, M.-C., Moon, J.-S., Miyata, T., Kong, Y.-Y.: Mind bomb 1-experssing intermediate progenitors generate Notch signaling to maintain radial glial cells. Neuron 58, 519-531 (2008)
Sunabori, T., Tokunaga, A., Nagai, T., Sawamoto, K., Okabe, M., Miyawaki, A., Matsuzaki, Y., Miyata, T., Okano, H.: Cell-cycle-specific nestin expression coordinates with morphological changes in embryonic cortical neural progenitors. J. Cell Sci. 121, 1204-1212 (2008)
Koyasu T, Kondo M, Miyata K, Ueno S, Miyata T, Nishizawa Y, Terasaki H. Photopic electroretinograms of mGluR6-deficient mice. Curr Eye Res. 33, 91-99 (2008)
Miyata, T.: Development of three-dimensional architecture of the neuroepithelium: Role of pseudostratification and cellular 'community'. Dev. Growth Differ. 50, S105-S112 (2008)
Sakaue-Sawano, A., Kurokawa, H., Morimura, T., Hanyu, A., Hama, H., Osawa, H., Kashiwagi, S., Fukami, K., Miyata, T., Miyoshi, H., Imamura, T., Ogawa, M., Masai, H. and Miyawaki, A.: Visualizing spatiotemporal dynamics of multicellular cell-cycle progression. Cell 132, 487-498 (2008).
Konno, D., Shioi, G., Shitamukai, A., Mori, A., Kiyonari, H., Miyata, T. and Matsuzaki, F.: Neuroepithelial progenitors undergo LGN-dependent planar divisions to maintain self-renewability during mammalian neurogenesis. Nat. Cell Biol. 10, 93-101 (2008)
Nishizawa, Y., Imafuku, H., Saito, K., Kanda, R., Kimura, M., Minobe, S., Miyazaki, F., Kawakatsu, S., Masaoka, M., Ogawa, M. and Miyata, T.: Survey of the morphogenetic dynamics of the ventricular surface of the developing mouse cortex. Dev. Dyn. 236, 3061-3070 (2007)
Tamai, H., Shinohara, H., Miyata, T., Saito, K., Nishizawa, Y., Nomura, T. and Osumi, N.: Pax6 transcription factor regulates interkinetic nuclear movement in cortical progenitor cells via centrosomal stabilization. Genes Cells 12, 983-996 (2007)
Miyata, T.: Morphology and mechanics of daughter cells "delaminating" from the ventricular zone of the developing neocortex. Cell Adh. Migr. 1, 99-101(2007)
Miyata, T., and Ogawa, M.: Twisting of neocortical progenitor cells underlies a spring-like mechanism for daughter cell migration. Curr.Biol. 17, 146-151 (2007)
Ochiai, W., Minobe, S., Ogawa, M., Miyata, T.: Transformation of pin-like ventricular zone cells into cortical neurons. Neurosci. Res. 57, 326-329 (2007)
Miyata, T.: Asymmetric cell division during brain morphogenesis. Prog. Mol. Subcell. Biol. 452, 121-142 (2007)
Hirai, S., Cui, DF., Miyata, T., Ogawa, M., Kiyonari, H., Suda, Y., Aizawa, S., Banda, Y. and Ohno, S.: The c-Jun N-terminal kinase activator dual leucine zipper kinase regulates axon growth and neuronal migration in the developing cerebral cortex. J. Neurosci. 26, 11992-12002 (2006)
Imai, F., Hirai, S., Akimoto, K., Koyama, H., Miyata, T., Ogawa, M., Noguchi, S., Sasaoka, T., Noda, T., and Ohno, S.: Inactivation of aPKCλ results in the loss of adherens junctions in neuroepithelial cells without affecting neurogenesis in mouse neocortex. Development 133, 1735-1744 (2006)
Mutoh, T., Miyata, T., Kashiwagi, S., Miyawaki, A., and Ogawa, M.: Dynamic behavior of individual cells in developing organotypic brain slices revealed by the photoconvertable protein Kaede. Exp. Neurol. 200, 430-437 (2006)
Naruse, M., Nakahira, E., Miyata, T., Hitoshi, S., Ikenaka, K., and Bansai, R.: Induction of oligodendrocyte progenitors in dorsal forebrain by intraventricular microinjection of FGF-2. Dev. Biol. 60, 1084-1100 (2006)
Zou, P., Muramatsu, H., Miyata, T., and Muramatsu, T.: Midkine, a heparin-binding growth factor, is expressed in neural precursor cells and promotes their growth. J. Neurochem. 99, 1470-1479 (2006)
Miyata, T., Saito, K., Nishizawa, Y., Murayama, A., Masaoka, M., and Ogawa, M.: Modern slice culture for direct observation of production and migration of brain neurons. Nagoya J. Med. Sci. 67, 65-70 (2005)
Ueno S, Kondo M, Miyata K, Hirai T, Miyata T, Usukura J, Nishizawa Y, Miyake Y. Physiological function of S-cone system is not enhanced in rd7 mice. Exp Eye Res. 81, 751-758 (2005)
Uematsu J, Nishizawa Y, Hirako Y, Kitamura K, Usukura J, Miyata T, Owaribe K. Both type-I hemidesmosomes and adherens-type junctions contribute to the cell-substratum adhesion system in myoepithelial cells. Eur J Cell Biol. 84, 407-415 (2005)
Kawaguchi, A., Ogawa, M., Saito, K., Matsuzaki, F., Okano, H., and Miyata, T.: Differential expression of Pax6 and Ngn2 between pair-generatged cortical neurons. J. Neurosci. Res. 78, 784-795 (2004)
Miyata, T., Kawaguchi, A., Saito, K., Kawano, M., Muto, T., and Ogawa, M.: Asymmetric production of surface-dividing and non-surface-dividing cortical progenitor cells. Development 131, 3133-3145 (2004)
Saito, K., Kawaguchi, A., Kashiwagi, S., Yasugi, S., Ogawa, M., and Miyata, T.: Morphological asymmetry in dividing retinal progenitor cells. Develop. Growth & Differ. 45, 219-229 (2003)
Shinozaki, K., Miyagi, T., Yoshida, M., Miyata, T., Ogawa, M., Aizawa, S., and Suda, Y.: Absence of Cajal-Retzius cells and subplate neurons associated with defects of tangential migration from ganglionic eminence in Emx1/2 double mutant cerebral cortex. Development 129, 3479-3492 (2002)
Miyata, T., Kawaguchi, A., Saito, K., Kuramochi, H., and Ogawa, M.: Visualization of cell cycling by an improvement in slice culture methods. J. Neurosci. Res.69, 861-868 (2002)
Ogawa Y, Sawamoto K, Miyata,T., Miyao S, Watanabe M, Nakamura M, Bregman BS, Koike M, Uchiyama Y, Toyama Y, Okano H.: Transplantation of in vitro-expanded fetal neural progenitor cells results in neurogenesis and functional recovery after spinal cord contusion injury in adult rats. J. Neurosci. Res. 69, 925-933 (2002).
Takasawa K, Kitagawa K, Yagita Y, Sasaki T, Tanaka S, Matsushita K, Ohstuki T, Miyata T, Okano H, Hori M, Matsumoto M. Increased proliferation of neural progenitor cells but reduced survival of newborn cells in the contralateral hippocampus after focal cerebral ischemia in rats. J. Cereb. Blood Flow Metab. 22, 299-307 (2002).
Yagita Y, Kitagawa K, Sasaki T, Miyata, T., Okano H, Hori M, Matsumoto M. Differential expression of Musashi1 and nestin in the adult rat hippocampus after ischemia. J. Neurosci. Res. 69, 750-756 (2002).
Yamazaki, Y., Makino, H., Hamaguchi-Hamada, K., Hamada, S., Sugino, H., Kawase, E., Miyata, T., Ogawa, M., Yanagimachi. R., and Yagi, T.: Assessment of the developmental totipotency of neural cells in the cerebral cortex of mouse embryo by nuclear transfer. Proc. Natl. Acad. Sci. USA S 98, 14022-14026 (2001)
Miyata, T., Kawaguchi, A., Okano, H., and Ogawa, M. Asymmetric inheritance of radial glial fibers by cortical neurons. Neuron 31, 727-741 (2001)
Kawaguchi, A., Miyata, T., Sawamoto, K., Takashita, N., Murayama, A., Akamatsu, W., Ogawa, M., Okabe, M., Tano, Y., Goldman, S.A., and Okano, H. Nestin-EGFP mice: visualization of the self-renewal and multipotency of CNS stem cells. Mol. Cell. Neurosci. 17, 259-273 (2001)
Yagita, Y., Kitagawa, K., Otsuki, T., Kuwabara, K., Mabuchi, T., Miyata, T., Okano, H., Hori, M., and Matsumoto, M.: Proliferation of neuronal progenitor cells and increased neurogenesis in the ischemic adult rat hippocampus. Stroke 32, 1890-1896 (2001)
Kaneko, Y., Sakakibara, S., Imai, T., Suzuki, A., Nakamura, Y., Sawamoto, K., Ogawa, Y., Toyama, Y., Miyata, T., and Okano, H.: Musashi1: an evolutionally conserved marker for CNS progenitor cells including neural stem cells. Dev. Neurosci. 22, 139-153 (2000)
Nakamura, Y., Sakakibara, S., Miyata, T., Ogawa, M., Shimazaki, T., Weiss, S., Kageyama, R., and Okano, H.: The bHLH gene Hes1 as a repressor of neuronal commitment of the CNS stem cells. J. Neurosci. 20, 283-293 (2000)
Ohtani, T., Ishihara, K., Atsumi, T., Nishida, K., Keneko, Y., Miyata, T., Itoh, S., Narimatsu, M., Maeda, H., Fukada, T., Itoh, M., Okano, H., Hibi, T., and Hirano, T.: Dissection of signaling cascade through gp130 in vivo: Reciprocal roles for STAT3-and SHP2-mediated signals in cytokine and immunoglobulin production. Immunity 12, 95-105 (2000)
Miyata, T., Maeda, T., and Lee, J.E.: NeuroD is required for differentiation of the granule cells in the cerebellum and hippocampus. Genes Dev. 13, 1647-1652 (1999)
Miyata, T., Nakajima, K., Mikoshiba, K., and Ogawa, M.: Regulation of Purkinje cell alignment by Reelin as revealed with CR-50 antibody. J. Neurosci. 17, 3599-3609 (1997)
Miyata, T., Nakajima, K., Mikoshiba, K., and Ogawa, M.: Distinct arrangement patterns of Purkinje cells between normal and reeler mice are reproduced in cerebellar explants. Dev. Neurosci. 19, 124 (1997)
Del Rio, J., Heimrich, B., Borrell, V., Froster, E., Drakew, A., Alcantara, S., Nakajima, K., Miyata, T., Ogawa, M., Mikoshiba, K., Derer, P., Frotscher, M., and Soriano, E.: A role for Cajal-Retzius cells and reelin in the development of hippocampal connections. Nature 385, 70-74 (1997)
Nakajima, K., Mikoshiba, K., Miyata, T., Kudo, C., and Ogawa, M.: Disruption of hippocampal development in vivo by CR-50 mAb against Reelin. Proc. Natl. Acad. Sci. USA 94, 8196-8201 (1997)
De Vergeyck, V., Nakajima, K., Lambert de Rouvroit, C., Naerhuyzen, B., Goffinet, A. M., Miyata, T., Ogawa, M., and Mikoshiba, K.: A truncated Reelin protein is produced but not secreted in the “Orleans” reeler mutation. Mol. Brain Res. 50, 85-90 (1997)
D’Arcangelo, G., Nakajima, K., Miyata, T., Ogawa, M., Mikoshiba, K., Curran, T.: Reelin is a secreted glycoprotein recognized by the CR-50 monoclonal antibody. J. Neurosci. 17, 23-31 (1997)
Yoneshima, H., Nagata, E., Matsumoto, M., Yamada, M., Nakajima, K., Miyata, T., Ogawa, M., and Mikoshiba, K.: A novel neurological mutant mouse, yotari, which exhibits reeler-like phenotype but expresses CR-50 antigen/Reelin. Neurosci. Res. 29, 217-223 (1997)
Miyata, T., Nakajima, K., Aruga, J., Takahashi, S., Ikenaka, K., Mikoshiba, K, and Ogawa, M.: Distribution of a reeler gene-related antigen in the developing cerebellum: an immunohistochemical study with an allogeneic antibody CR-50 on normal and reeler mice. J Comp. Neurol. 372, 215-228 (1996)
Sakakibara, S., Okano, H., Imai, T., Hamaguchi, K., Aruga, J., Nakajima, K., Nagata, T., Kurihara, Y., Uesugi, S., Miyata, T., Ogawa, M., and Mikoshiba, K.: Mouse-musashi-1, a neural RNA-binding protein highly enriched in the mammalian CNS stem cell. Dev. Biol. 176, 230-242 (1996)
Takahashi, S., Yamamoto, H., Matsuda, Z., Ogawa, M., Yagyu, K., Taniguchi, T., Miyata, T., Koda, H., Higuchi, T., Okutani, F., and Fujimoto, S.: Identification of two highly homologous presynaptic proteins distinctly localized at the dendritic and somatic synapses. FEBS letters 368, 455-460 (1995)
Ogawa, M., Miyata, T., Nakajima, K., Yagyu, K., Ikenaka, K., Yamamoto, H., and Mikoshiba, K.: The reeler gene-associated antigen on Cajal-Retzius neurons is a crucial molecule for laminar organization of cortical neurons. Neuron 14, 899-912 (1995)
Miyata, T., and Ogawa, M.: Developmental potentials of early telencephalic neuroepithelial cells: a study with microexplant culture. Dev. Growth & Differ.36, 319-331 (1994)